ULTIMAKER GAINS CERTIFICATION AS PART OF MATERIALISE 510(K) CLEARED MIMICS INPRINT SOFTWARE

Furthering Ultimaker’s dedication to reliability and accessibility, we’re excited to announce that the Ultimaker S5 has been certified by Materialise as a 3D printing solution to create orthopedic, maxillofacial, and cardiovascular models for clinical use when used in combination with Mimics inPrint software. The company partnered with Materialise to identify compatibility of the Ultimaker S5 with Materialise Mimics inPrint software to meet FDA regulatory guidelines for point-of-care 3D printing in hospitals.
What does this certification mean?
While a growing number of free and commercial medical imaging software is available, in some cases within the United States, models marketed for diagnostic use must be prepared using software cleared by the FDA, such as Materialise Mimics inPrint.
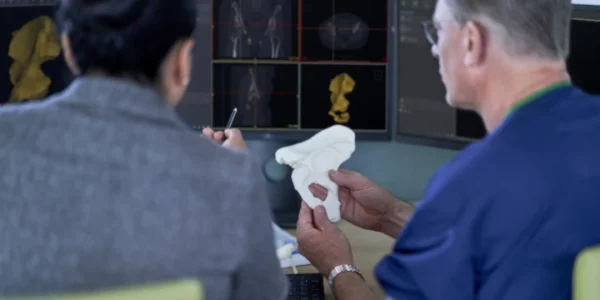
Revolutionizing patient care
With the continued adoption of 3D printing for treatment planning and diagnostics, hospitals are recognizing that tangible, anatomically correct models make it easier to diagnose patients, plan surgeries, and discuss treatment where they would otherwise need to rely on 2D images. “The Materialise certification of the 3D printing workflow when used with Mimics inPrint reduces the safety and quality-control burden on doctors and hospitals with its clearance by the FDA,” says John Kawola, President of Ultimaker North America.
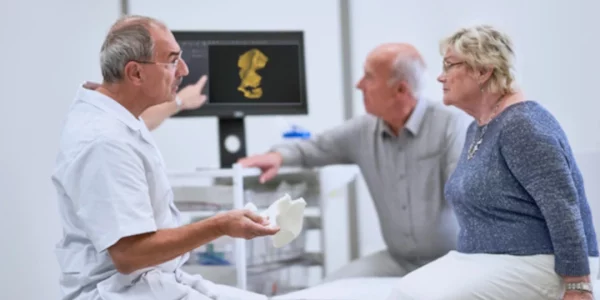
If you’re interested in learning more, read the report below.
Read the Materialise report
Learn more about the Ultimaker S5 model:

